If a radical mechanism is operative in a gi ven reaction and if an alkene pi bond is present in the molecule an intramolecular radical addition reaction may be observed.
Why is the vinyl radical unstable.
Understand the chemical and physical properties of free radicals along with the examples important uses and the sources for extracting free radicals.
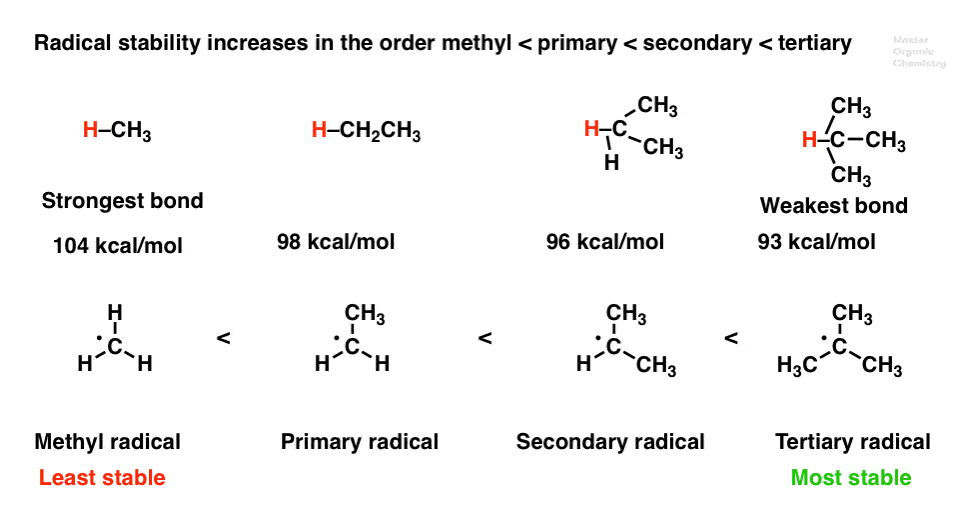
Radicals increase in stability in.
The instability derives from the inability of that p orbital to overlap with the the sp2 orbitals of the carbon on the other end of the double bond.
Radical probes carbon centered radicals as well as many other types of radicals show a propensity for addition to carbon carbon pi bonds.
This is usually due to steric factors the reactive free radical center is surrounded by bulky groups and just not sterically accessible for reaction or electronic factors some free radicals exist in very large delocalized systems hence the spin density at any one atom in the system is so small that reaction is unlikely.
The fluoromethyl radical you cite is indeed less stable than the corresponding methyl radical but this is because the shape of the radical goes from planar ish to more pyramidal meaning that the radical is more sigma in character than pi.
A free radical has an unpaired electron that has the highest energy among all bonding and non bonding electrons in a molecule.
What are free radicals.
A vinyl cation is a positively charged molecule a cation where the positive charge is located on a vinyl group ch ch2.
Conclusion the configurational stability of a vinyl anion can be understood from the relative instability of intermediate 7 in its inversion process whereas the corresponding intermediate in the inversion of a vinyl radical 10 is relatively more stable leading to a lower energy barrier to inversion.
When you combine the lumo of an ewg with the somo of a radical you get a lowering of the somo energy and therefore stabilisation.
If the carbon with the double bond has a radical it is unstable if a methyl substituent of the vinyl has the radical it is stable due to conjugation resonance.
The hybridization of a vinyl carbocation is sp hybirdized.
Vinyl carbocation is unstable.